


The Rapid Response™ RSV Test Cassette is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab and nasal aspirate specimens in children less than 6 years and adults over the age of 60. The test is intended for use as an aid in the rapid laboratory diagnosis of acute respiratory syncytial virus infection in patients with symptoms consistent with RSV infection.
The Rapid Response™ RSV Test Cassette is CLIA Waived and FDA Cleared for Prescription in vitro diagnostic use only.
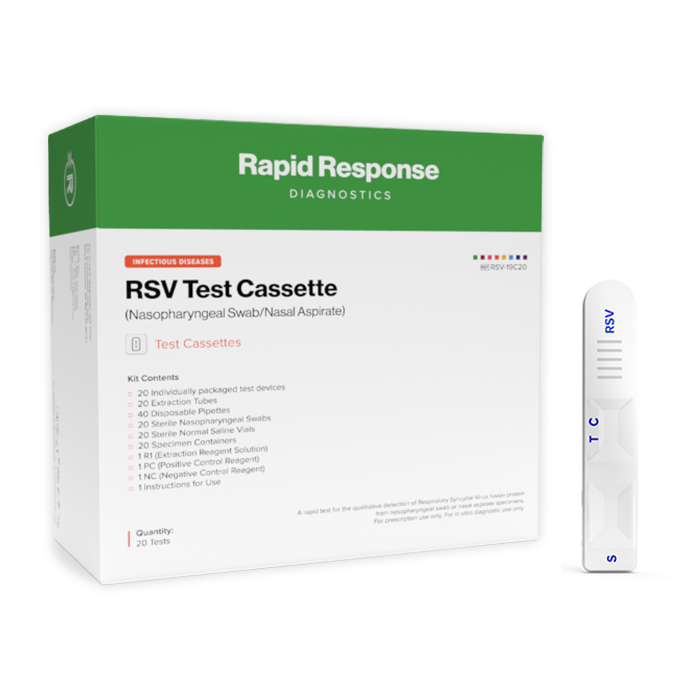
- 20 Individually packaged test devices
- 20 Sterile Nasopharyngeal swabs
- 20 Specimen processing tubes
- 20 Sterile normal saline vials
- 20 Extraction tubes
- 40 Disposable pipettes
- 1 R1 (Extraction reagent solution)
- 1 PC (Positive control reagent)
- 1 NC (Negative control reagent)
- 1 Instructions for use
The Rapid Response™ RSV Test Cassette is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab and nasal aspirate specimens in children less than 6 years and adults over the age of 60. The test is intended for use as an aid in the rapid laboratory diagnosis of acute respiratory syncytial virus infection in patients with symptoms consistent with RSV infection.
The Rapid Response™ RSV Test Cassette is CLIA Waived and FDA Cleared for Prescription in vitro diagnostic use only.
Aids in the diagnosis of acute RSV infection
Read results in 15 minutes
For nasopharyngeal swab or nasal aspirate samples
CLIA Waived for prescription in vitro diagnostic use only
Respiratory syncytial virus is a highly contagious, acute, viral infection of the respiratory tract. The causative agent is a single stranded (negative strand) RNA virus of the Paramyxoviridae family. RSV is now recognized as the leading cause of hospitalization of children during the first year of life. It is also the major viral cause of nosocomial illness in children already hospitalized for other reasons. Half of all infants become infected during their first year of life. Virtually all have been infected by their second year.1 Infection involving the lower respiratory tract carries an associated mortality rate of 0.5%, especially in premature infants or infants and children with underlying lung disease.
.png)
Please refer to the product insert for more details.

The BTNX Rapid Response Tests undergo regular evaluation using proficiency samples from CAP (College of American Pathologists).
- Respiratory Syncytial Virus Activity — United States 2000-01 MMWR, CDC January 18, 2002.
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.

