This product includes drug parameters and cut-off levels, with options that may vary for forensic, point-of-care, or laboratory professional use settings. Certain product configurations are CLIA waived and/or FDA cleared. Please contact us for details and regional availability.
The Rapid Response™ Multi-Drug Cassette is a rapid, qualitative test for the simultaneous detection of multiple drugs and drug metabolites in human urine. It is intended for in vitro diagnostic use only.
Custom combinations of up to 14 drug parameters are available contact info@lochnessmedical.com to learn more.
- Test Cassettes
- Droppers
- Product Insert
This product includes drug parameters and cut-off levels, with options that may vary for forensic, point-of-care, or laboratory professional use settings. Certain product configurations are CLIA waived and/or FDA cleared. Please contact us for details and regional availability.
The Rapid Response™ Multi-Drug Cassette is a rapid, qualitative test for the simultaneous detection of multiple drugs and drug metabolites in human urine. It is intended for in vitro diagnostic use only.
Custom combinations of up to 14 drug parameters are available contact info@lochnessmedical.com to learn more.
Compact format for easy storage
Pipette included with each individually packaged cassette
Control (C) and Test (T) regions marked on cassette
The Rapid Response™ Multi-Drug Test Cassette (Urine) is a one-step immunoassay in which chemically labeled drugs (drug-protein conjugates) compete for limited antibody binding sites with drugs which may be present in urine. The test device contains membrane strips which are pre-coated with drug-protein conjugates on the test band(s). Each strip, the drug antibody-colloidal gold conjugate pad is placed at one end of the membrane. In the absence of drug in the urine, the solution of the colored antibody-colloidal gold conjugate moves along with the sample solution upward chromatographically by capillary action across the membrane to the immobilized drug-protein conjugate zone on the test band region. The colored antibody-gold conjugate then attaches to the drug-protein conjugates to form visible lines as the antibody complex with the drug conjugate. Therefore, the formation of the visible precipitant in the test zone occurs when the test urine is negative for the drug. When the drug is present in the urine, the drug/metabolite antigen competes with the drug-protein conjugate on the test band region for the limited antibody. When a sufficient concentration of the drug is present, it will fill the limited antibody binding sites. This will prevent attachment of the colored antibody (drug-protein conjugate)-colloidal gold conjugate to the drug-protein conjugate zone on the test band region. Therefore, absence of the color band on the test region indicates a positive result.
A control band with a different antigen/antibody reaction is added to the immunochromatographic membrane strip at the control region (C) to indicate that the test has performed properly. This control line should always appear regardless of the presence of a drug or metabolite. If the control line does not appear, the test device should be discarded.
.png)
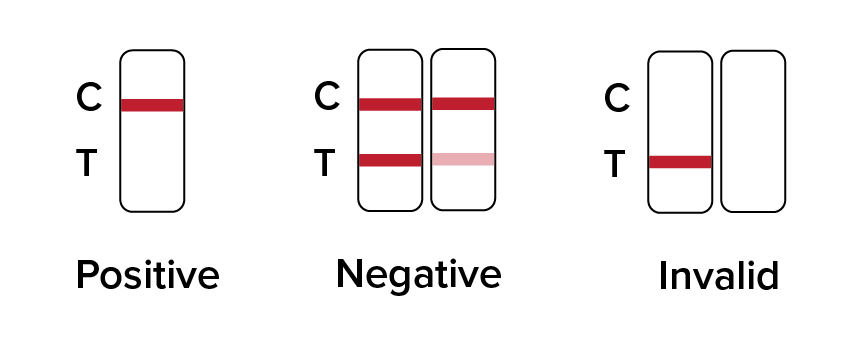
Positive: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
Negative: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
Invalid: NO line appears in the C – Control region, then the result is Invalid.
Please refer to the product insert for more details.
This product is also available with an option of built-in Specimen Validity/Adulteration Test Strips (DX.X-1F29). The following parameters to check for adulteration are available:
- Oxidants/Pyridinium Chlorochromate
- Specific Gravity
- pH
- Nitrite
- Glutaraldehyde
- Creatinine
Adulteration is the tampering of a urine specimen with the intention of altering the test results. The use of adulterants can cause false negative results in drug tests by either interfering with the screening test and/or destroying the drugs present in the urine. Dilution may also be employed in an attempt to produce false negative drug test results.
Urine Collection: The Rapid Response™ Multi-Drug Test Cassette (Urine) is designed for use with urine specimens. Fresh urine does not require any special handling or pretreatment. The urine specimen must be collected in a clean and dry container. Urine collected at any time of the day may be used. Urine specimens exhibiting visible precipitates should be centrifuged, filtered, or allowed to settle to obtain clear specimen for testing.
Urine Storage: It is recommended the collected fresh urine to be tested immediately. Fresh urine may be stored at room temperature (25ºC) for up to 4 hours or refrigerated (2-8ºC) for up to 48 hours prior to performing the test. For prolonged storage, specimens may be frozen and stored below -20ºC. Specimens that have been refrigerated must be brought to room temperature prior to testing. Previously frozen specimens must be thawed, brought to room temperature, and mixed thoroughly prior to testing.
NOTE: Urine specimens and all materials coming in contact with them should be handled and disposed of as if capable of transmitting infection. Avoid contact with skin by wearing gloves and proper laboratory attire.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.