This product is compatible with our Lateral Flow Scanner. Streamline your drug screening workflow, record test results of up to 5 drug screens, and transmit results to your ATS or EMR. Watch a demo here!














This product includes drug parameters and cut-off levels, with options that may vary for forensic, over-the-counter, point-of-care, or laboratory professional use settings. Certain product configurations are CLIA waived and/or FDA cleared. Please contact us for details and regional availability.
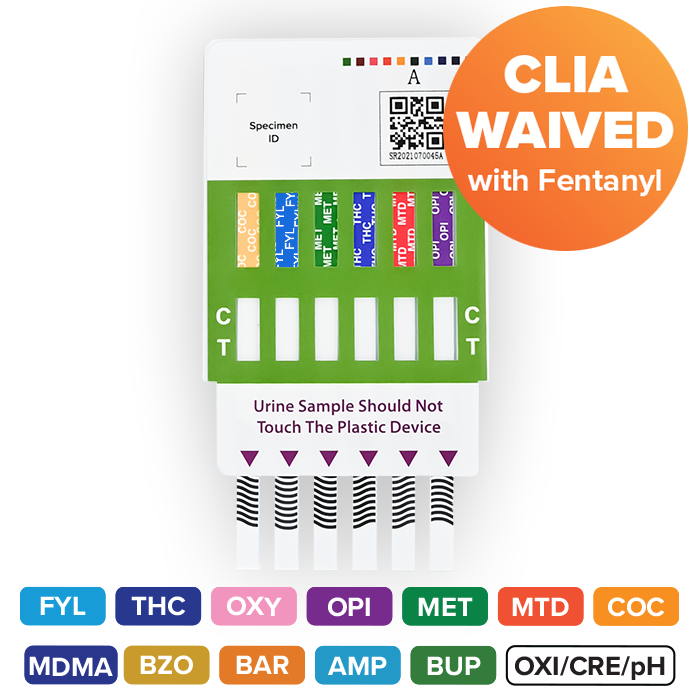
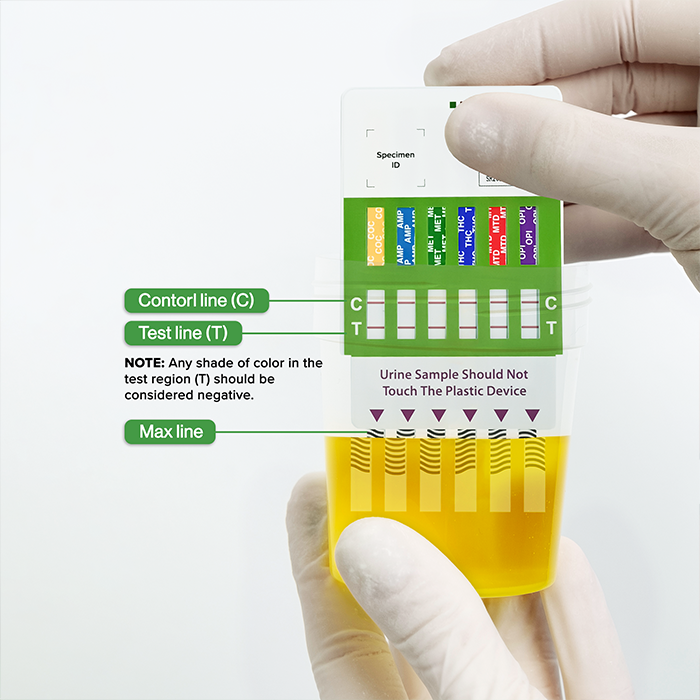
The Rapid Response™ Multi-Drug Test Panel is a one step, qualitative test for the simultaneous detection of multiple drugs and metabolites in human urine. In just 5 minutes, test results can be observed through the visual interpretation of coloured lines.
Our Multi-Drug Test Panels are customizable and allows for combinations of up to 16 parameters with SAMHSA recommended drug testing cut-offs. Built-in Specimen Validity/Adulteration Test Strips are also available at request!
Custom parameter combinations of 1-16 drugs available. Contact info@lochnessmedical.com for more information.

RapidReader App

Lateral Flow Scanner
- Test Panels
- SVT/Adulterant Color Chart (if applicable)
- Product Insert
This product includes drug parameters and cut-off levels, with options that may vary for forensic, over-the-counter, point-of-care, or laboratory professional use settings. Certain product configurations are CLIA waived and/or FDA cleared. Please contact us for details and regional availability.
The Rapid Response™ Multi-Drug Test Panel is a one step, qualitative test for the simultaneous detection of multiple drugs and metabolites in human urine. In just 5 minutes, test results can be observed through the visual interpretation of coloured lines.
Our Multi-Drug Test Panels are customizable and allows for combinations of up to 16 parameters with SAMHSA recommended drug testing cut-offs. Built-in Specimen Validity/Adulteration Test Strips are also available at request!
Custom parameter combinations of 1-16 drugs available. Contact info@lochnessmedical.com for more information.
Test up to 14 parameters simultaneously
Control and Test Regions marked for easy interpretation
Integrated adulteration testing options available
Longer detection window than saliva tests
Fentanyl testing available!
The Rapid Response Multi-Drug Test Panel is available with an option of built-in Specimen Validity/ Adulteration Test Strips (DX.X-1B29-25). The following parameters are available:
- Oxidants/ Pyridinium Chlorochromate
- Specific Gravity
- pH
- Nitrite
- Glutaraldehyde
- Creatinine
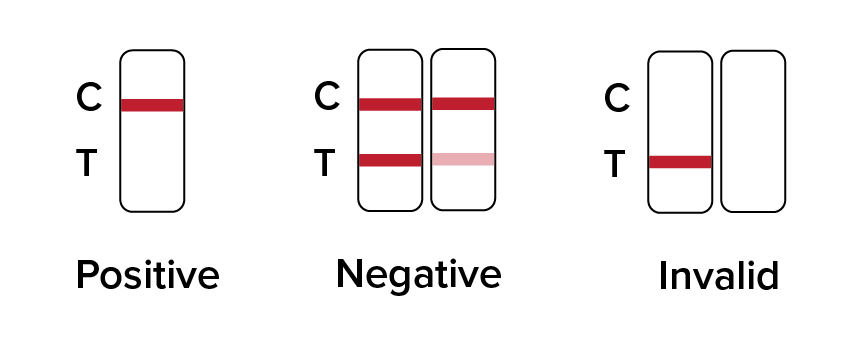
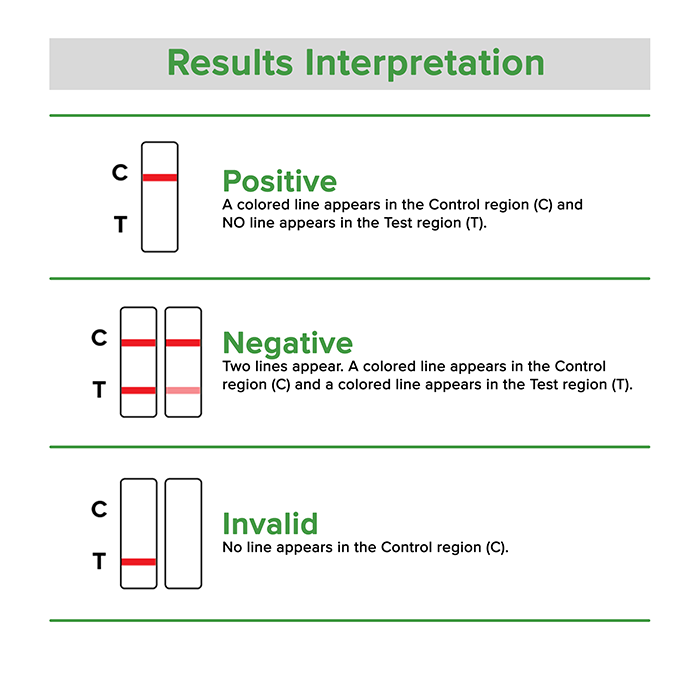
Results Interpretation are as follows:
Positive: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
Negative: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
Invalid: NO line appears in the C – Control region, then the result is Invalid.
Please refer to the product insert for more details.
The Rapid Response™ Multi-Drug Test Panel is compatabile with the Rapid Response™ Lateral Flow Scanner, a revolutionary workflow solution redefining the approach to drug screen reporting in staffing agencies, treatment centers, and laboratories. The Lateral Flow Scanner captures results of up to 5 test panels, allows the operator to confirm the results and then transmits the data directly to your organizations EMR, ATS or LIS with the click of a button.
The BTNX Rapid Response Tests undergo regular evaluation using proficiency samples from AAB (American Association of Bioanalysts) and CAP (College of American Pathologists).
Urine Storage: It is recommended the collected fresh urine to be tested immediately. Fresh urine maybe stored at room temperature (25ºC) for up to 4 hours or to be refrigerated (2-8ºC) for up to 48 hours prior to performing the test. For prolonged storage, specimens may be frozen and stored below -20ºC. Specimens that have been refrigerated must be brought to room temperature prior to testing. Previously frozen specimens must be thawed, brought to room temperature, and mixed thoroughly prior to testing.
NOTE: Urine specimens and all materials coming in contact with them should be handled and disposed of as if capable of transmitting infection. Avoid contact with skin by wearing gloves and proper laboratory attire.
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.
